Alle Produkte werden in einer diskreten Verpackung verschickt. Es gibt keinen Hinweis auf unser Geschäft oder unsere Produkte auf der Verpackung

Alle Produkte werden in einer diskreten Verpackung verschickt. Es gibt keinen Hinweis auf unser Geschäft oder unsere Produkte auf der Verpackung

Alle Produkte werden in einer diskreten Verpackung verschickt. Es gibt keinen Hinweis auf unser Geschäft oder unsere Produkte auf der Verpackung

Alle Produkte werden in einer diskreten Verpackung verschickt. Es gibt keinen Hinweis auf unser Geschäft oder unsere Produkte auf der Verpackung

Alle Produkte werden in einer diskreten Verpackung verschickt. Es gibt keinen Hinweis auf unser Geschäft oder unsere Produkte auf der Verpackung

Alle Produkte werden in einer diskreten Verpackung verschickt. Es gibt keinen Hinweis auf unser Geschäft oder unsere Produkte auf der Verpackung

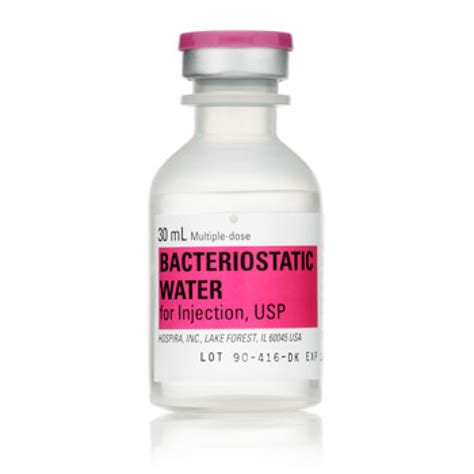



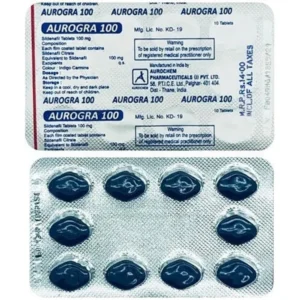


Bewertungen
Es gibt noch keine Bewertungen.